Recently, Prof. Wen-Jing Xiao and co-workers have made significant progress on visible-light-induced enantioselective aerobic oxidation. The results have been published in the top international authoritative academic journal “Journal of the American Chemical Society” (J. Am. Chem. Soc. 2017, 139, 63-66. IF=13.038). Central China Normal University is the only corresponding institution.
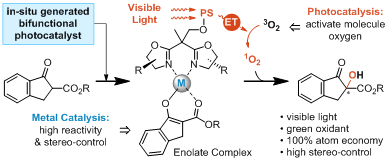
Over the past decade, asymmetric photocatalysis induced by visible light has flourished as a powerful tool for the sustainable, highly efficient, and selective production of enantioenriched compounds. However, asymmetric visible light photocatalysis has mainly relied on the use of two separate catalysts, an achiral photocatalyst and a chiral cocatalyst, in a single organic transformation. A single chiral photocatalyst that is able to induce a stereoselective photochemical reaction would be advantageous but is challenging, because it must possess the following two functions: harvest visible light or sunlight to activate the reagents and control the stereoselectivity during the chemical bond formation. Therefore, the design and invention of new bifunctional chiral photocatalysts for efficient asymmetric photochemical transformations is highly desirable to enable new reactivity.
In this study, Xiao and co-workers have successfully developed a novel family of visible-light-responsive chiral ligands by grafting a triplet state photosensitizer to chiral bisoxazoline ligands. Complexation of this ligand with Ni(acac)2 results in a powerful catalyst for the asymmetric oxidation reaction of β-ketoesters, which uses oxygen or air as the green oxidant and visible light or sunlight as the ideal driving force. Using this protocol, products containing the α-hydroxy-β-dicarbonyl motif are produced in high yields and with excellent enantiopurities.
The work was financially supported by the National Natural ScienceFoundation of China (Nos. 21232003, 21472057, 21472058, and 21572074).