Recently, Prof. Wenjing Xiao, Prof. Liangqiu Lu and coworkers hasmade significant progress on Copper catalysed heterocycle synthesis. Theresults have been published in the top international authoritative academicjournal “Angewandte Chemie Intertnational Edition” (Angew. Chem. Int. Ed. 2016,55, 12422-12426. IF =11.709). Central China Normal University is the onlycorresponding institution
Rapid access to valuable heterocycles remains a subject ofintensive research in chemical synthesis. In this context, indole has been apopular platform to develop new methodologies. In the past decade,transition-metal-catalyzed reaction sequences of aniline-bearing propargylalcohols and their derivatives have been demonstrated to be a highly efficientand fruitful strategy for the construction of functionalized indoles; however,most of the reported methods require the use of catalysts based on precious transitionmetals, i.e., gold, rhodium, platinum, or palladium.
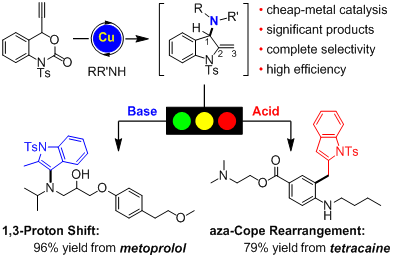
In this study, Xiao and Lu developed a copper-catalyzeddecarboxylative amination/hydroamination sequence of propargylic carbamateswith various nucleophiles. Simple switch of the reaction condition allows thedivergent synthesis of two types of functionalized indoles with highefficiencies and complete chemoselectivities. Importantly, experiments todemonstrate the synthetic potential of this methodology were performed. Thiswork not only provides key precursors for natural alkaloid synthesis and othervaluable complex molecules, but also realizes the modification of someprescribed drugs (e.g., tetracaine, metoprolol, and duloxetine), demonstratingthe potential to discover new pharmaceutical candidates.
The work was financially supportedby the National Natural ScienceFoundation of China (21232003, 21472057 and 21572074) and other financialsupports (NO. 201422, CCNU15A02007 and 2015CFA033).